Introduction
The demand for environmentally friendly and multifunctional textile materials has made massive contributions to the advancement of the modifications in textile fabrics (1). Cotton is one of the most used natural fibers, characterized by softness and breathability (2). Nevertheless, it is sensitive to staining as well as microbial growth due to a high degree of hydrophilicity (3, 4). Among a wide range of ideas, the involvement of nanomaterials is one of the most promising pathways for improving the sustainability of the fabrics while maintaining their properties (5). Recently, researchers have been especially interested in utilizing semiconductor metal oxides in the nano regime that have specific photocatalytic properties, through which they have the ability to degrade organic compounds in the presence of light irradiation (6). The recent research has proven that various methods, like the utilization of nanoparticles that enhance the functional properties of fabrics, can be utilized to develop self-cleaning fabrics. For instance, it has been proved through studies how efficiently silver nanoparticles and titanium dioxide (TiO2) provide cotton fabrics with antibacterial and self-cleaning properties (7, 8). Additionally, applications of hybrid nanomaterials, such as TiO2-Ag-ZnO composites, have been studied to enhance photocatalysis and broaden light absorption ranges, making them more effective under visible light conditions (9). This investigation of self-cleaning properties for cotton fabric coated with nano photocatalyst is designed to develop a textile not only maintaining its inherent properties but also contributing to environmental sustainability (10). Through the photocatalysis process, the organic stains that are attached to the cotton fabrics are degraded into less toxic small fragments (11). Furthermore, the amount of water required for stain removal can be reduced drastically and these coatings support environmental sustainability (12). For photocatalysis self-cleaning behavior, photocatalyst, like zinc oxide nanoparticles (ZnO NPs) is widely used owing to its affordability, accessibility, and facile synthesis procedure (13). ZnO NPs is an n-type semiconductor with a broad bandgap (∼3.3 eV) at room temperature, allowing them to absorb photons in the near UV wavelength to excite the electrons (14). Recently, reports were validating that the synthesis route and the concentration of ZnO NPs influence the photocatalytic performance, with higher concentration generally leading to improved performance (15). Though low concentrations of elemental Zn are necessary for cellular processes and metabolism, high concentrations can lead to toxicity (16). Therefore, ZnO NPs can be coated only in low concentrations to reduce the risk of detachment from the surface of the fabric, which may lead to toxicity. In contrast, reports suggested that if the ZnO NPs are coated over fabrics in situ, the toxicity was declined as the detachment of ZnO NPs from the fabrics was restricted owing to physical attachment (17). The in situ approach prevents ZnO nanowires from separating from the cloth and improves long-term performance by ensuring strong adhesion. Reducing the demand for chemical detergents and water during cleaning utilizing ZnO nanowire enables a sustainable environment. The following characteristics reflects the potential of ZnO nanowires in advancing eco-friendly textile technologies. This work addresses the fabrication of cotton fabrics with ZnO nanowires without using advanced methods for efficient stain removal and environmental sustainability. Energy dispersive X-ray (EDX) spectroscopy mapping analysis, field emission scanning electron microscopy (FESEM), and X-ray diffraction (XRD) further validated the presence of ZnO nanowires in the fabric. Artificial white LED light is used for the stain degradation studies against methyl blue and methyl violet dyes.
Materials and method
Materials and chemicals required
The 100% pure commercially available cotton fabric and 1200 W white LED light were sourced from the local market. Zinc sulfate heptahydrate (ZnSO4.7H2O) and sodium hydroxide (NaOH) were obtained from Merk, India.
In-situ growth of ZnO nanowires over cotton fabric
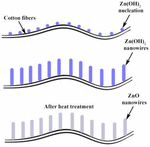
Solution A and solution B were prepared by dissolving 0.56 g of ZnSO4.7H2O in 50 ml of distilled water and 0.8 g of NaOH in 50 ml of distilled water, respectively. The sample (i.e., cotton fabric) was immersed in solution A under constant stirring, solution B was added dropwise to solution A, followed by an aging process for 2 h. During the course of time, nucleation and crystal growth of Zn(OH)2-occurred over the surface of the cotton fibers, leading to the formation of nanowires (Figure 1). Afterward, the Zn(OH)2 coated fabric sample was taken out from the solution and washed with ethanol 2–3 times. The washed fabric was then placed on Petri plates and treated in a hot air oven overnight at 60°C. The dried Zn(OH)2-coated fabric was kept in a muffle furnace for 1 h at 150°C. During dehydration, Zn(OH)2 was converted to ZnO nanowires. The chemical reactions involved are,
Photocatalytic self-cleaning activity
The self-cleaning behavior of fabric was enabled by the presence of photocatalytic ZnO nanowires over its surface and therefore, the stain removal behavior of the fabric was estimated against model dyes. Two samples of coated and ZnO uncoated fabrics were taken and placed on the petri plate. To avoid the folding of the samples, the samples were stuck using double side adhesive tapes. 0.01 g of dyes (methyl blue and methyl violet) were dissolved in 100 ml, respectively, labeled as stock solution and wrapped with aluminum foil to avoid the light interaction. From the stock solution, 2 ml of dyes were taken and added to 8 ml of distilled water. Using the 2 ml Pasteur pipette, two drops of dyes were placed over the coated and uncoated fabrics causing them to absorb and get stained by dyes. Now, the samples were placed under the 1200 W white LED light and photographs were taken every 5 min to follow the decolorization effect through photocatalysis process.
Characterization techniques
The structural features of ZnO nanowires coated fabrics were characterized using XRD (D8 Advance ECO, Bruker, Germany). The surface morphologies of coated and uncoated cotton fabrics were studied using FESEM (SUPRA-55, Carl Zeiss, Germany). The elemental composition of the ZnO nanowires-coated fabrics was analyzed using EDX spectrum and EDX mapping.
Results and discussion
XRD analysis
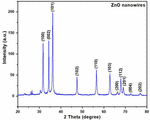
The XRD pattern of ZnO nanowires was recorded in the 2θ range of 20°–80° (Figure 2). The diffraction pattern displayed 2θ peaks at 31.73°, 34.38°, 36.26°, 47.48°, 56.56°, 62.82°, 66.27°, 67.89°, 69.01°, 72.46°, and 76.88°, corresponding to the Miller indices (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), and (202), respectively, confirmed from the ICDD card no. #00–036-1451 (18). From the diffraction peaks, it is elucidated that the ZnO nanocrystals were formed even at the low calcination temperature of 150°. Further, the crystal structure of ZnO nanowires was confirmed as a wurtzite hexagonal structure (19).
FESEM analysis
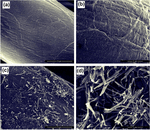
The topographical morphology of the ZnO-coated and uncoated fabrics was revealed using FESEM images. The uncoated fabric displayed a smooth fiber surface without any impurities or dust (Figure 3a and b). However, after coating with ZnO nanowires, the surface turned rough with numerous nanowires that had grown over the surface (Figure 3c and d). The length of each nanowire was evaluated to be 2–3 μm with a thickness of 50–100 nm, having an aspect ratio of 1:30–1:40.
EDX spectrum
The EDAX spectrum for ZnO-coated fabric was recorded to elucidate the presence of Zn over the fiber surface. The EDX spectrum for the area scanned at 5 μm (Figure 4a) and 10 μm (Figure 4b) displayed that elemental Zn was present over both the scanned area of the ZnO-coated fabric. Furthermore, the EDX mapping spectra were evaluated to check whether elemental Zn was uniformly distributed over the coated fabric (Figure 4c). The EDX spectrum of Zn L confirmed that the elemental Zn was found uniformly over the surface of the cotton fibers and has Zn-dense regions where the ZnO nanowires were found dense. The relatively lower atomic percentage of Zn compared to O observed in the EDX spectrum is due to the fact that ZnO is coated on cotton fabric, which has excess carbon (C) and oxygen (O). This inherent composition of the cotton fabric adds more to the percentage of oxygen in the EDX analysis compared to the actual expected ZnO composition.

Figure 4. EDX spectra of ZnO nanowires coated cotton fabric scanned at different areas (a) 5 μm, (b) 10 μm and (c) EDX mapping spectra of ZnO nanowires coated cotton fabric.
Photocatalytic activity
The ZnO nanowires coated cotton fabrics were subjected to self-cleaning studies. The coated and uncoated fabrics were stained with methyl blue and methyl violet dyes and were kept under 1200 W white LED light. Photographs were taken at regular intervals of 5 min and shown in Figure 5. From the photographs, it was clear that after 45 min of exposure, the ZnO nanowires-coated fabric completely removed the stains caused by dyes, whereas the stains in the uncoated fabrics were still intact with a slight reduction in the intensity, which may be caused due to photosensitization process. The stain removal or decolorization of dyes by coated samples was primarily attributed to the presence of ZnO nanowires, which are well known for their photocatalytic activity. Further, as the photosensitization process was seen in the uncoated samples, it will also be occurring in the stain removal in the coated samples but enhanced in the presence of ZnO nanowires.

Figure 5. Photographs demonstrating the self-cleaning behavior of the ZnO nanowires coated cotton fabric.
Mechanism of photocatalysis
The photocatalytic degradation of dyes is caused by the reactive oxygen species (ROS) generated during the photosensitization and photocatalysis process enabled through photocatalyst, i.e., ZnO nanowires. The photosensitization process involves exciting dye molecules with the irradiation of photons with the required wavelength. The dye’s excited electrons (e) instead of recombining are shifted to the conduction band of ZnO nanowires. The harvested e–s reacted with oxygen to generate superoxide radicals () which in turn attack the dye molecules and degraded them, causing decolorization. The photocatalytic degradation process involves the generation of photon-induced e–/h+ pairs in the ZnO nanowires. The photo-excited e–s reacted with oxygen to generate , and the h+s reacted with OH– to form hydroxyl radicals (OH*). These ROS interact with the dye molecules and facilitate the photocatalytic degradation process.
Considerations on toxicity
The potential toxicity is a major factor when ZnO nanowires are used as a coating on self-cleaning fabrics. Research shows that ZnO nanoparticle exposure can cause respiratory problems and skin irritation, among other health hazards. Although too high elemental zinc can be harmful, low quantities are vital for many biological activities (20, 21). Previous studies demonstrating both acute and chronic impacts of ZnO exposure emphasize the requirement of safe handling and application techniques. On the other hand, ZnO nanowires in situ preparation improves their adhesion to fabrics, thereby lowering the possibility of detachment and hence the toxicity hazards (22, 23). Future research will focus on studies of stability and toxicity to handle these issues.
Comparative analysis with literature data
To validate the significance of the present study, literature data are compared with the results obtained through the present study and are summarized in Table 1. From the table, it is inferred that there are many reports that state the self-cleaning behavior of ZnO nanomaterial-coated fabrics. However, the efficiency of the photocatalytic activity primarily depends on the size and shape of the photocatalyst. In this regard, the ZnO nanowires grown in situ over the cotton fabric in the present study display higher efficiency compared to ZnO having shapes, such as flowers, spheres, and nanorods. This comparative analysis substantiates the better activity and an improvement in the field of ZnO-based self-cleaning coating for cotton fabric.
Conclusion
This study demonstrates cotton fabrics coated with ZnO nanowires that have self-cleaning properties and impart stain resistance properties to cotton fabric. The in situ growth of ZnO nanowires was demonstrated by several characterization techniques such as XRD, FESEM, and EDX. The photocatalytic activity of ZnO-coated fabric was evaluated from the degradation of organic dyes, particularly methyl blue and methyl violet, by 1200 W white LED light exposure. Results indicate that the ZnO-coated cotton fabric was able to totally remove the stains of dyes within 45 min compared to an untreated fabric, where the reduction in the intensity of stains was minimal. The self-cleaning ability is enhanced due to the ROS generated from the ZnO nanowires, hence degrading the organic pollutants on the surface of the fabric. The results indicate that ZnO nanowires are an effective photocatalyst for self-cleaning purposes and demonstrate the environmental sustainability of such coatings. The use of ZnO-coated cotton fabrics can reduce the necessity of water and chemical detergents in cleaning, promising a solution for the eco-friendly applications of textiles. On the other hand, different limitations of this study should be considered. Practical uses depend on assessing long-term stability and durability of ZnO nanowires on the cloth. The toxicity level of the ZnO nanowires, particularly in terms of the long-term exposure, needs to be assessed. While the efficacy of ZnO-coated fabric against a wider range of stains and pollutants has yet to be assessed.
References
1. Zhang, Y, Xia, X, Ma, K, Xia, G, Wu, M, Cheung, YH, et al. Functional textiles with smart properties: their fabrications and sustainable applications. Adv Funct Mater. (2023) 33:2301607.
2. Ferrándiz, M, Fages, E, Rojas-Lema, S, vorra-Martinez, JI, Gomez-Caturla, J, and Torres-Giner, S. Development and characterization of weft-knitted fabrics of naturally occurring polymer fibers for sustainable and functional textiles. Polymers (Basel). (2021) 13:665.
3. Sfameni, S, Lawnick, T, Rando, G, Visco, A, Textor, T, and Plutino, MR. Functional silane-based nanohybrid materials for the development of hydrophobic and water-based stain resistant cotton fabrics coatings. Nanomaterials. (2022) 12:3404.
4. Tan, Y, Fang, K, Chen, W, Shi, Q, and Zhang, C. Fabrication of a superhydrophobic cotton fabric with efficient antibacterial properties and asymmetric wettability via synergistic effect of quaternized chitosan/TiO2/Ag. Ind Crops Prod. (2024) 209:118034.
5. Shah, MA, Pirzada, BM, Price, G, Shibiru, AL, and Qurashi, A. Applications of nanotechnology in smart textile industry: a critical review. J Adv Res. (2022) 38:55–75.
6. Lanjwani, MF, Tuzen, M, Khuhawar, MY, and Saleh, TA. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: a review. Inorg Chem Commun. (2024) 159:111613.
7. Girigoswami, A, Deepika, B, Pandurangan, AK, and Girigoswami, K. Preparation of titanium dioxide nanoparticles from Solanum Tuberosum peel extract and its applications. Artif Cells Nanomed Biotechnol. (2024) 52:59–68.
8. Jaast, S, and Grewal, A. Green synthesis of silver nanoparticles, characterization and evaluation of their photocatalytic dye degradation activity. Curr Res Green Sustain Chem. (2021) 4:100195.
9. Tjardts, T, Elis, M, Hicke, M, Symalla, F, Shondo, J, Drewes, J, et al. Critical assessment of a TiO2-Ag-ZnO nanocomposite photocatalyst on improved photocatalytic activity under mixed UV-visible light. Appl Surf Sci Adv. (2023) 18:100500.
10. Mushtaq, Q, Awan, T, Momna, M, Amjad, M, Intisar, A, and Afzal, A. Sustainable self-cleaning fabrics enabled by sunlit metal oxide catalysts: a critical review. Sustain Mater Technol. (2024):e01009.
11. Yang, J, He, T, Li, X, Wang, R, Wang, S, Zhao, Y, et al. Rapid dipping preparation of superhydrophobic TiO2 cotton fabric for multifunctional highly efficient oil-water separation and photocatalytic degradation. Coll Surf A Physicochem Eng Asp. (2023) 657:130590.
12. Jiang, X, Tian, X, Gu, J, Huang, D, and Yang, Y. Cotton fabric coated with nano TiO2-acrylate copolymer for photocatalytic self-cleaning by in-situ suspension polymerization. Appl Surf Sci. (2011) 257: 8451–6.
13. Lee, TX. Applications and Future Perspectives of Photocatalytic Coatings for Air Purification and Self Cleaning. Final Year Project, UTAR (2022).
14. Norek, M. Approaches to enhance UV light emission in ZnO nanomaterials. Curr Appl Phys. (2019) 19:867–83.
15. Mirzaei, A, Yerushalmi, L, Chen, Z, and Haghighat, F. Photocatalytic degradation of sulfamethoxazole by hierarchical magnetic ZnO@ g-C3N4: RSM optimization, kinetic study, reaction pathway and toxicity evaluation. J Hazard Mater. (2018) 359:516–26.
16. Ali, A, Phull, A-R, and Zia, M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol Rev. (2018) 7:413–41.
17. Javed, A, Azeem, M, Wiener, J, Thukkaram, M, Saskova, J, and Mansoor, T. Ultrasonically assisted in situ deposition of ZnO nano particles on cotton fabrics for multifunctional textiles. Fibers Polym. (2021) 22:77–86.
18. Tamanna, NJ, Hossain, MS, Bahadur, NM, and Ahmed, S. Green synthesis of Ag2O & facile synthesis of ZnO and characterization using FTIR, bandgap energy & XRD (Scherrer equation, Williamson-Hall, size-train plot, Monshi-Scherrer model). Results Chem. (2024) 7:101313.
19. Somveer, F, Saini, GS, and Kumari, M. Investigating structural changes of ZnO nanoparticles using powder X-ray diffraction over 6 months. Int J Stat Appl Math (2023) SP-8(3):630–4.
20. Girigoswami, K, Viswanathan, M, Murugesan, R, and Girigoswami, A. Studies on polymer-coated zinc oxide nanoparticles: UV-blocking efficacy and in vivo toxicity. Mater Sci Eng C. (2015) 56:501–10.
21. Girigoswami, A, Deepika, B, Udayakumar, S, Janani, G, Mercy, DJ, and Girigoswami, K. Peony-shaped zinc oxide nanoflower synthesized via hydrothermal route exhibits promising anticancer and anti-amyloid activity. BMC Pharmacol Toxicol. (2024) 25:101.
22. Tănase, MA, Soare, AC, Oancea, P, Răducan, A, Mihăescu, CI, Alexandrescu, E, et al. Facile in situ synthesis of ZnO flower-like hierarchical nanostructures by the microwave irradiation method for multifunctional textile coatings. Nanomaterials. (2021) 11:2574.
23. Tan, LY. Functionalization of Cotton Fabric with ZnO/PVA for Antibacterial Textile Application. Malaysia: Universiti Tunku Abdul Rahman (2018).
24. Lawrynowicz, A, Palo, E, Nizamov, R, and Miettunen, K. Self-cleaning and UV-blocking cotton–fabricating effective ZnO structures for photocatalysis. J Photochem Photobiol A Chem. (2024) 450:115420.
25. Zhu, C, Shi, J, Xu, S, Ishimori, M, Sui, J, and Morikawa, H. Design and characterization of self-cleaning cotton fabrics exploiting zinc oxide nanoparticle-triggered photocatalytic degradation. Cellulose. (2017) 24:2657–67.
26. Kumbhakar, P, Pramanik, A, Biswas, S, Kole, AK, Sarkar, R, and Kumbhakar, P. In-situ synthesis of rGO-ZnO nanocomposite for demonstration of sunlight driven enhanced photocatalytic and self-cleaning of organic dyes and tea stains of cotton fabrics. J Hazard Mater. (2018) 360:193–203.
27. Tania, IS, Ali, M, and Akter, M. Fabrication, characterization, and utilization of ZnO nanoparticles for stain release, bacterial resistance, and UV protection on cotton fabric. J Eng Fiber Fabr. (2022) 17:15589250221136378.
28. Ashraf, M, Champagne, P, Perwuelz, A, Campagne, C, and Leriche, A. Photocatalytic solution discoloration and self-cleaning by polyester fabric functionalized with ZnO nanorods. J Ind Text. (2015) 44:884–98.
© The Author(s). 2024 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.